In the boardroom of any global healthcare entity, a new and formidable challenge has taken center stage. It isn’t just the pace of clinical innovation or the rising cost of labor; it is regulatory divergence. For decades, the goal of the global healthcare industry was harmonization, a steady march toward unified standards that would allow a medical device, a pharmaceutical product, or a digital health platform to scale seamlessly across borders. Today, that trend has reversed. We have entered an era of “protectionist compliance,” in which data sovereignty, localized AI ethics, and fragmented supply-chain mandates are the new reality.
In this landscape, compliance can no longer be a reactive check-the-box function buried in the legal department. To survive the next decade, healthcare leaders must transform compliance into a strategic engine of resilience.
The Data Sovereignty Trap: Beyond Simple Privacy
The first and perhaps most significant shift in the global regulatory landscape is the transition from Data Privacy to Data Sovereignty. While the world spent the last several years adapting to the General Data Protection Regulation (GDPR) and its many international clones, the goalposts have moved. It is no longer sufficient to protect patient data; regulators are increasingly requiring that health data remain within national borders. From the European Health Data Space (EHDS) to strict localization laws in emerging markets such as India and Indonesia, the borderless cloud is eroding.
The Strategic Risk
For global healthcare providers and tech firms, this creates an interoperability trap. If a clinical trial relies on a centralized database in North America but enrolls patients in Southeast Asia or the EU, the organization may face compliance paralysis, unable to move the data needed to improve patient outcomes without violating local laws.
The Solution: Decentralized Architecture
The winners in this space are moving away from monolithic, centralized data lakes. Instead, they are investing in federated or edge computing models. These enable localized data storage that complies with sovereignty laws while using global queries to extract insights without moving the underlying sensitive information across a digital border.
The AI Frontier: Governance as the New Clinical Standard
As Artificial Intelligence (AI) moves from experimental pilot projects to the backbone of clinical decision support and administrative automation, a new regulatory frontier has emerged: Algorithmic Accountability.
We are seeing a global patchwork of AI regulation. The European Union’s AI Act treats healthcare as a high-risk sector, demanding rigorous transparency and human oversight. Simultaneously, other regions are taking a more “innovation-first” approach, creating a minefield for companies trying to deploy a single global AI tool.
The Problem of Clinical Bias
Regulators are no longer just assessing whether an AI works; they are also evaluating what it was trained on. A diagnostic algorithm trained on a specific demographic in Western Europe may be flagged as non-compliant or even dangerous if deployed in Africa or South America without localized validation.
Building Audit-Ready AI
To navigate this shift, organizations must move toward Explainable AI (XAI). If a regulator asks why a machine recommended a specific treatment path or denied a claim, the black box said so, is no longer a legally defensible answer. Establishing a global AI Ethics Board and implementing Human-in-the-Loop protocols aren’t just a moral choice; they’re a prerequisite for market access.

Supply-chain Transparency: The Ethical Mandate
The global pandemic exposed the fragility of healthcare supply chains, but the regulatory response has focused more on ethics than on logistics. New mandates on ESG (Environmental, Social, and Governance) and human rights are forcing healthcare companies to examine their tier-two and tier-three suppliers more closely. Due diligence regulations such as the German Supply Chain Due Diligence Act (LkSG) and the EU’s Corporate Sustainability Due Diligence Directive (CSDDD) hold manufacturers legally responsible for the environmental and labor practices of suppliers of raw materials for their surgical instruments or medication packaging.
From Efficiency to Resilience
The old model of Just-in-Time global shipping is being replaced by Just-in-Case localized manufacturing. Governments are incentivizing (and in some cases, requiring) near-shoring or friend-shoring of essential medical goods. For the worldwide strategist, this means your compliance team must now be as proficient in maritime law and carbon accounting as they are in clinical safety.
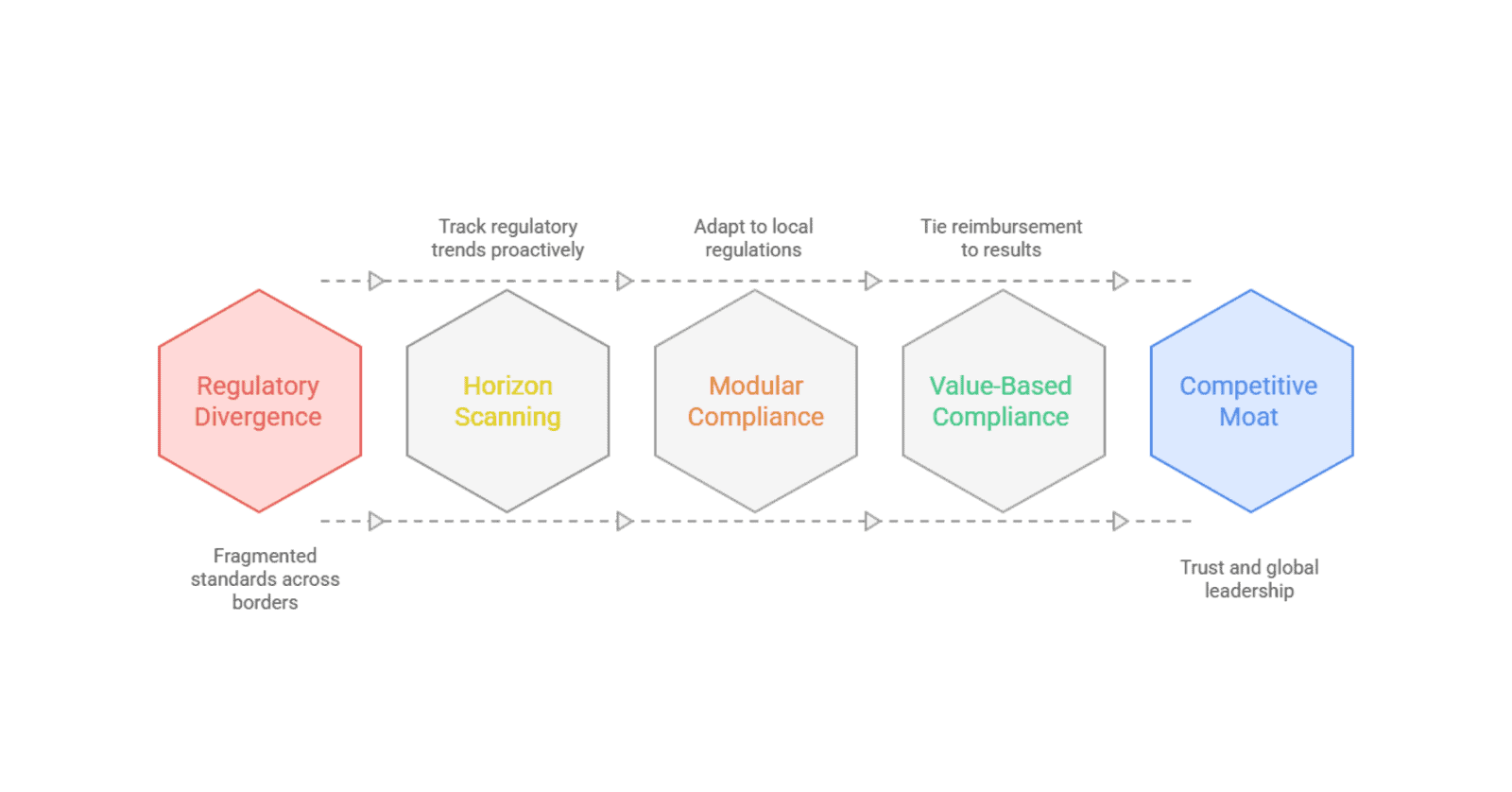
Value-Based Compliance: Tying Reimbursement to Results
Perhaps the most profound shift is the global movement from Volume to Value. Whether it is the Value-Based Healthcare (VBHC) initiatives in the Nordics or the outcome-based reimbursement models emerging in the Middle East, regulators are changing the definition of a “successful” healthcare transaction.
The Compliance Pivot
In a volume-based world, compliance focuses on preventing upcoding or over-utilization. In a value-based world, the risk shifts toward under-utilization or data manipulation. If your reimbursement is tied to patient recovery rates, the accuracy and integrity of your clinical data become your primary compliance risk.
Regulators are increasingly using sophisticated data analytics to flag anomalies in patient outcome reporting. Transparency is no longer a PR buzzword; it is a financial necessity.
The Global Roadmap: A 3-Step Strategy for Resilience
How does a global healthcare organization stay ahead of a target that is constantly moving? It requires a fundamental restructuring of the compliance function.
Shift to Horizon Scanning
Most organizations respond to laws only after they are passed. Strategic leaders invest in Regulatory Intelligence. By tracking white papers from the WHO, draft legislation in the EU, and policy shifts in the G20, you can identify regulatory trends 18 to 24 months before they become enforceable. This lead time is the difference between a smooth transition and a panicked, expensive pivot.
Adopt Modular Compliance
Stop trying to build a single Global Compliance Manual. Instead, create a Modular Framework. Your Core should consist of universal ethical standards and high-level quality controls. Additionally, you plug in Local Modules for specific regions, a GDPR module for Europe, a HIPAA module for the US, and an NMPA module for China. This allows the organization to remain agile without reinventing the wheel for every new market entry.
Compliance as a Valuation Driver
In the world of Healthcare M&A, Compliance Due Diligence is now the most scrutinized part of the deal. A company with clean regulatory standing across multiple jurisdictions is worth significantly more than one with high growth but high regulatory risk. Treat your compliance program as an asset that enhances your organization’s enterprise value.
Turning Complexity into a Moat
The global healthcare landscape is not going to get simpler. The days of one product, one planet are over. However, this complexity presents a significant opportunity.
Complexity is a barrier to entry. For the organization that masters the art of navigating these shifts, regulatory divergence isn’t a headache; it’s a competitive moat. By staying ahead of data residency laws, leading the way in AI ethics, and embracing transparency, you don’t just remain compliant. You build the trust necessary to lead the next generation of global healthcare.
The cost of transformation is high, but the cost of standing still is the loss of your global footprint. It’s time to stop fearing the shift and start navigating it.